Abstract
Introduction CT-P10 is a rituximab biosimilar approved by regulatory agencies in many countries including European Union[1], the United States[2], and the Republic of Korea[3]. This Korean post-marketing surveillance (PMS) study evaluated the safety and effectiveness of CT-P10 in the firstly approved indications in Korea: non-Hodgkin's lymphoma (NHL), chronic lymphocytic leukemia (CLL), rheumatoid arthritis (RA), granulomatosis with polyangiitis (GPA), and microscopic polyangiitis (MPA). In the CT-P10 development program, randomized controlled trials (RCTs) were conducted in RA and follicular lymphoma (FL). Therefore, the present PMS study would provide a comprehensive profile of CT-P10 in indications where approval was based on the extrapolation of comparative clinical data. Moreover, this study would address the real-world data of CT-P10 in patients with a range of comorbidities and taking various concomitant medications during routine clinical practice.
Method This was a prospective, open-label, observational PMS study conducted at 27 centers in Korea during the surveillance period (16 November 2016-15 November 2020). Patients who received their first CT-P10 treatment per prescribing information were eligible for the study, regardless of previous treatment receiving other rituximab products. The objective was to investigate the type and frequency of unexpected adverse events (AEs), adverse drug reactions (ADRs), and serious AEs during a 1-year (NHL and CLL) or 24-week (RA and GPA/MPA) follow-up period after the first administration of CT-P10. Effectiveness was assessed by treating physicians according to the best overall response (BOR) during the study in NHL and CLL patients, Disease Activity Score in 28 joints (DAS28) score in RA patients, and Birmingham Vasculitis Activity Score for Wegener's Granulomatosis (BVAS/WG) criteria in GPA/MPA patients following the first CT-P10 administration.
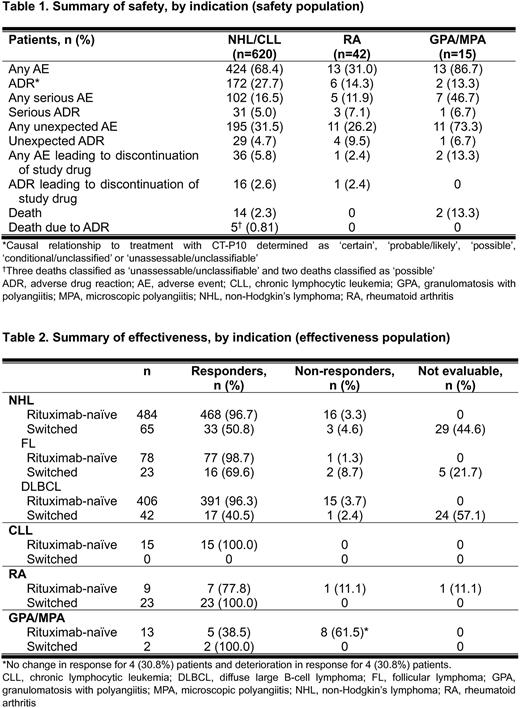
Results The safety population was defined as patients who received ≥1 dose of CT-P10 and had ≥1 safety follow-up visit after the first CT-P10 administration, and comprised 677 patients (604 NHL, 16 CLL, 42 RA, 15 GPA/MPA). Among 604 NHL patients, 108 patients were diagnosed with FL and 496 patients had diffuse large B-cell lymphoma (DLBCL). 611 patients (549 NHL, 15 CLL, 32 RA, 15 GPA/MPA) were included in the effectiveness population, excluding 66 patients whose effectiveness results were not reported.
Of 620 NHL or CLL patients, 424 (62.8%) experienced 1,717 AEs and 172 (27.7%) had ADRs (Table 1). The most common AE and ADR was nausea in NHL or CLL patients, reporting for 69 (11.1%) patients and 30 (4.8%) patients, respectively. Of 42 RA patients, 13 (31.0%) patients reported a total of 35 AEs. Eight ADRs occurred in 6 (14.3%) RA patients. 13 (86.7%) out of 15 GPA/MPA patients reported a total of 45 AEs. The most common AE was cough (3 [7.1%]) in RA patients and pneumonia (4 [26.7%]) in GPA/MPA patients, respectively.
In NHL patients, the response rate was 96.7% (468/484) for rituximab-naïve patients and 50.8% (33/65) for switched patients (i.e. who had received other rituximab products before CT-P10 treatment) each (Table 2). All CLL patients were rituximab-naïve and responder. The response rate was 77.8% (7/9) for rituximab-naïve patients and 100.0% (23/23) for switched patients in RA population. In GPA/MPA patients, response rates were 38.5% (5/13) and 100.0% (2/2) for rituximab-naïve and switched patients, respectively.
Conclusion This PMS study demonstrated that the safety findings of CT-P10 were consistent with the known safety profile of CT-P10 and other rituximab products in NHL, CLL, GPA, MPA and RA. In terms of effectiveness analyses, high response rates were observed across indications, providing pertinent information for the use of CT-P10 in routine clinical practice.
Reference [1] European Medicines Agency. Truxima summary of product characteristics 2022
[2] US Food and Drug Administration. Highlights of prescribing information, Truxima (CT-P10). 2022
[3] Ministry of Food and Drug Safety. Biological products 2016
Disclosures
Yoon:Kirin Pharm: Honoraria, Research Funding; Janssen Pharmaceuticals: Honoraria, Research Funding; Roche: Honoraria; Sanofi: Research Funding; Celltrion: Honoraria, Research Funding; Beigene: Research Funding; Boryung pharmaceutical: Honoraria, Research Funding; SAMYANG Biopharm: Honoraria, Research Funding; Pharos iBio: Consultancy; Ab clone: Consultancy; Amgen: Honoraria; BMS: Honoraria; Novartis: Honoraria; GI cell: Consultancy; Takeda: Honoraria; GSK: Honoraria; Celgene: Honoraria; GC Cell: Consultancy; AbbVie: Research Funding. Kim:Celltrion, Inc.: Current Employment. Ahn:Celltrion, Inc.: Current Employment. Park:Celltrion, Inc.: Current Employment. Park:Celltrion, Inc.: Current Employment. Han:Celltrion, Inc.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.